
![]()
Home Personnel Publications Research Jobs Links Photo Album
![]()
The first goal of my laboratory is to understand the biological basis of
disorders caused by dysfunction of the basal ganglia and use that knowledge to
develop novel therapies for this group of neurological diseases. Currently,
studies in my lab focus on a disease known as DYT1 dystonia, the most common
form of inherited dystonia. DYT1 dystonia, a dominantly inherited, incurable
disease, is caused by a three-nucleotide deletion in the gene TOR1A
that causes the loss of a glutamic acid in the protein torsinA. TorsinA is a AAA
protein (ATPases Associated with diverse cellular Activities) that
resides primarily in the endoplasmic reticulum. However, our lab and others
demonstrated that torsinA carrying the disease-causing mutation accumulates
abnormally in the nuclear envelope. 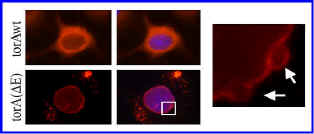
Over the next few years, my laboratory will focus on DYT1 dystonia research through the following major projects:
1) Define the biological role of torsinA and its role in DYT1pathogenesis
2) Understand how the neuronal protein quality control machinery “handles” torsinA and the
significance of this process in disease pathogenesis
3) Development of therapeutic RNAi interference for DYT1 dystonia. In addition to enhancing our
knowledge on the pathogenesis of dystonia and related disorders, these studies will contribute to
exploit RNA interference as a therapy for DYT1 and other incurable neurological diseases.
 A
second objective of our research is to understand the role of neuronal protein
quality control in aging. We have very recently started experiments aimed to
determine the causes and consequences of cellular aging or senescence. There is
experimental evidence indirectly implicating dysfunction of protein homeostasis
in aging. It has also been proposed that the nervous system plays an important
role in the regulation of this phenomenon. We hypothesize that progressive
age-related defects in neuronal protein homeostasis play an important role in
mammalian aging, both in a cell-autonomous and non-cell-autonomous manner. To
test this hypothesis, we are currently generating transgenic mice in which we
aim to accelerate neuronal aging through the manipulation of protein
homeostasis.
A
second objective of our research is to understand the role of neuronal protein
quality control in aging. We have very recently started experiments aimed to
determine the causes and consequences of cellular aging or senescence. There is
experimental evidence indirectly implicating dysfunction of protein homeostasis
in aging. It has also been proposed that the nervous system plays an important
role in the regulation of this phenomenon. We hypothesize that progressive
age-related defects in neuronal protein homeostasis play an important role in
mammalian aging, both in a cell-autonomous and non-cell-autonomous manner. To
test this hypothesis, we are currently generating transgenic mice in which we
aim to accelerate neuronal aging through the manipulation of protein
homeostasis.
![]()