Upcoming Change in HIV Testing
Effective Monday, November 23, 2015, the University of Iowa Hospitals and Clinics core clinical laboratory will switch the main Human Immunodeficiency Virus (HIV) screening test to the Bio-Rad GS HIV Combo Ag/Ab EIA, an automated FDA-approved assay that detects both antigen and antibody in patients infected with either HIV-1 (group M and O) or HIV-2. This will replace the Abbott Diagnostics’ HIV Antigen/Antibody (Ag/Ab) Combo assay.
The Epic name for the new assay is: HIV ANTIGEN/ANTIBODY COMBO (HIV-1 & HIV-2) [LAB7444].
This assay produces separate results for the following:
• HIV p24 antigen
• HIV-1 antibodies
• HIV-2 antibodies
Positivity for any combination of these three components is considered a positive result that reflexes to confirmation testing. Reactive specimens are tested in duplicate. In specimens with high titers of HIV-1 antibodies, the determination of p24 antigen may not be possible.
Below are examples of common patterns of results:
| HIV p24 antigen | HIV-1 antibodies |
HIV-2 antibodies |
Interpretation |
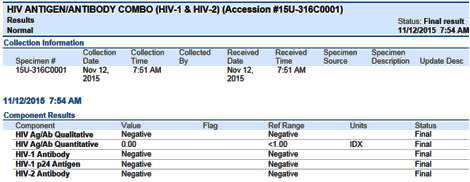
| Negative | Negative | Negative | Negative |
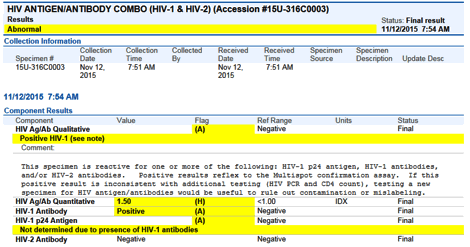
| Positive | Negative | Negative | Positive for HIV-1 |
| Positive | Positive | Negative | Positive for HIV-1 |
| Not determined due to presence of HIV-1 antibodies |
Positive | Negative | Positive for HIV-1 |
| Negative | Negative | Positive | Positive for HIV-2 |
The first line of each result will have the overall interpretation indicated. Screenshots of two example results are at the end of this broadcast.
The Combo assay will be available 24/7, 365 days a year (including holidays) with a turnaround time of two hours or less after specimen receipt in the laboratory. Specimen requirements are unchanged from previous combo assay. Positive results automatically reflex to the Multi-Spot confirmation assay.
Like many other HIV screening tests, the Combo assay is not approved for children less than two years old. For HIV screening in children less than two years, PCR testing is often used. Consultation with pediatric infectious disease specialists is recommended if unsure about testing approaches in children less than two years old.
“HIV QUANTITATIVE PCR” [Epic 2468, “viral load”] will continue to be available.
Questions should be directed to Matthew Krasowski, MD, PhD, Director of Clinical Laboratories (384-9380, matthew-krasowski@uiowa.edu).
Example screenshots:(1) Negative Result
(2) Positive for HIV-1 Antibodies (p24 antigen not determined)