Change in Estradiol Testing
Effective today, April 20, 2015, the University of Iowa Hospitals and Clinics core clinical chemistry laboratory will change the assay version for the serum/plasma estradiol assay (ESTRADIOL, Epic # LAB523). The current assay (Roche Diagnostics Estradiol II) will change to the next generation assay (Estradiol III).
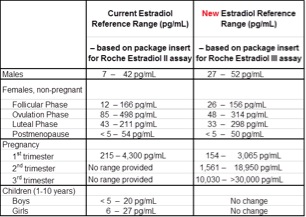
We will be updating reference ranges to those in the package insert for the Estradiol III assay. Normal ranges now include those for 2nd and 3rd trimester of pregnancy. Pediatric reference ranges do not change. There are no changes to specimen requirements or turnaround time.
Reference ranges for estradiol display only in the footnote to the assay results given the variability across menstrual cycle, pregnancy, menopause, and stages of puberty/development.
A summary of the reference range changes are summarized in the table below.
Questions should be directed to Matthew Krasowski, MD, PhD, medical director of the Clinical Chemistry Laboratory (384-9380, matthew-krasowski@uiowa.edu).