Rapid Group A Streptococcal testing, modifications
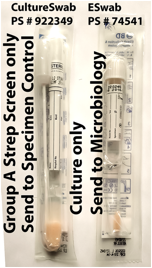
As of 6/19/2013, the University of Iowa Hospitals and Clinics have transitioned to ESwabs (PS #74541) for culture of aerobic, anaerobic, and fastidious bacteria. The Rapid Group A Strep screen is a non-culture-based method that requires the older aerobic CultureSwab (PS #922349, see picture).
The transition to ESwabs requires a modification to the protocol for Rapid Strep screens with parallel culture. Formerly, these panel tests (Group A Rapid Strep Screen Panel, Epic LAB1140; and Perianal Rapid Strep Screen Panel, Epic O51295) required two CultureSwabs. Now, one ESwab and one CultureSwab should be submitted. Send the ESwab and requisition for Group A Strep Culture to Microbiology and send the CultureSwab and requisition for Group A Rapid Strep Screen to Specimen Control.
CultureSwabs are stocked in OmniCells and clinics where Rapid Strep screening is frequently performed and are for Rapid Strep screening only. For culture, ESwabs should be used instead. Please contact Hospital Stores if CultureSwabs are necessary for Rapid Strep screening but are not available in your area.
Questions concerning this broadcast can be directed to Bradley Ford, MD, PhD, Associate Medical Director of Microbiology, (ext. 6-2990, bradley-ford-1@uiowa.edu).