Changes to High Risk HPV DNA Testing
Beginning Monday May 14, 2012, the University of Iowa Hospitals and Clinics Microbiology and Molecular Pathology Laboratory will implement Human Papillomavirus (HPV) types 16 and 18 genotyping and switch to a PCR method for high risk HPV detection using the Cobas HPV Test (Roche Diagnostics, Inc.). The high risk HPV group includes types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68 which are associated with cervical cancer and its precursor lesions.
- Several organizations including the American Cancer Society, American Society for Colposcopy and Cervical Pathology (ASCCP), and the American Society for Clinical Pathology recommend HPV16 and HPV18 genotype testing for women 30-65 years of age with normal cytology and who have a positive HPV high risk result (Saslo D, Journal of Lower Genital Tract Disease, Mar. 2012).
- The presence of HPV16 or HPV18 in these particular specimens is further indication for examination of the patient with colposcopy.
- If the results are negative for HPV16 and HPV18, the recommendation is to repeat cytology and HPV high risk DNA testing in one year.
- Following these guidelines, the laboratory will only report HPV types 16 and 18 genotype results for women ages 30-65 years old with a normal cytology or Pap test who have a positive high risk HPV PCR result.
In conjunction with these changes in HPV testing and in keeping with the current professional recommendations, the EPIC order (CON312 Consult Pathology-Cytology GYN Procedure for HPV testing in association with Pap testing) has been modified. Under step 9. High Risk HPV testing of this order, the listed reasons for testing will be reduced to the following three options:
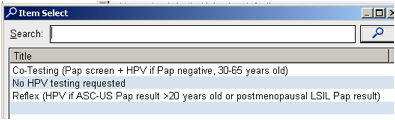
Co-testing refers to performing high risk HPV testing in addition to cytology if pap negative and patient is >30 years old or for reflex HPV testing for ASCUS or postmenopausal LSIL cytologic findings. Reflex testing refers to HPV testing for cytologic findings of ASCUS in women >21 years old or of LSIL in postmenopausal women.
HPV testing can also be ordered independent of the Pap test (EPIC order LAB7624). Stand-alone HPV testing may be most appropriate for follow up of patients with prior abnormal cervical cytology and/or histology per ASCCP guidelines.
The HPV PCR test has been validated by the laboratory for use with SurePath Liquid based Pap collection and tissue biopsy specimens.
Questions may be directed to Aaron Bossler, M.D., Ph.D. Molecular Pathology Laboratory (4-9566, pager 4703, aaron-bossler@uiowa.edu) or Chris Jensen, M.D., Cytopathology (6-3217, chris-jensen@uiowa.edu).