DHEA Sulfate Assay Now Available in Clinical Chemistry Laboratory
Effective Wednesday, May 9, 2012, the University of Iowa Hospitals and Clinics core clinical laboratory will begin running the Roche Diagnostics assay for dehydroepiandrosterone sulfate (DHEA sulfate). DHEA sulfate is typically ordered for workup of hyperadrenogenism (including polycystic ovarian syndrome), premature adrenarche, or as an adjunct in diagnosis of congenital adrenal hyperplasia. This assay will be available in Epic as "DEHYDROEPIANDROSTERONE SULFATE" [LAB524] and replace the current mailout test which uses the same methodology and units of measurement.
| •Specimen Type: | 4.5 mL light green plasma separator tube (PST) |
| •Minimum Requirements (Adult): | 3 mL whole blood from one light green plasma separator tube (PST) |
| •Minimum Requirements (Peds): | TWO light green top microtainer (pediatric patients) |
| •Test Availability: | 24 hours/day, 7 days a week (including holidays) |
| •Turnaround Time: | 1 hour upon receipt in laboratory |
| •Reference Ranges: | 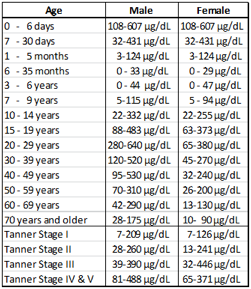 |
Questions should be directed to Matthew Krasowski, MD, PhD, medical director of the Clinical Chemistry Laboratory (384-9380, matthew-krasowski@uiowa.edu).